A radiopharmaceutical associates a radioisotope (as a payload) and a targeting agent (a small molecule or a biologic). This conjugate will target tumor cells for either diagnostic or therapeutic purposes. Mainly dedicated to oncology, these radiopharmaceuticals use either beta, alpha or gamma emitters (radioisotopes such as Lutetium-177, Yttrium-90, Indium-111..).
With a strong background in radioimmunotherapy, we propose our classic preclinical services (in vitro assays & DMPK) for the development of your radiopharmaceutical: cytotoxicity assay, internalization assay, pharmacokinetics, tissue distribution. Complementary investigations required for the specific development of radiopharmaceuticals are also available.
Immunoreactivity – Lindmo assay
Immunoreactivity is one of the most important quality controls of antibody-based radiopharmaceuticals. Determination of the immunoreactive fraction is a critical parameter that can be evaluated using cell-based assays. Measurement of immunoreactivity of a radiolabeled antibody is determined using linear extrapolation to binding at infinite excess (method of Lindmo et al.).
Efficacy studies
Our team can validate efficacy of any therapeutic radiopharmaceutical (Lutetium-177, Yttrium-90, Astatine-211, Actinium-225…). Efficacy and assessment of antitumor activity can be evaluated in a large variety of syngeneic and xenograft tumor models. We can use common tumor cell line or your own transfected cells.
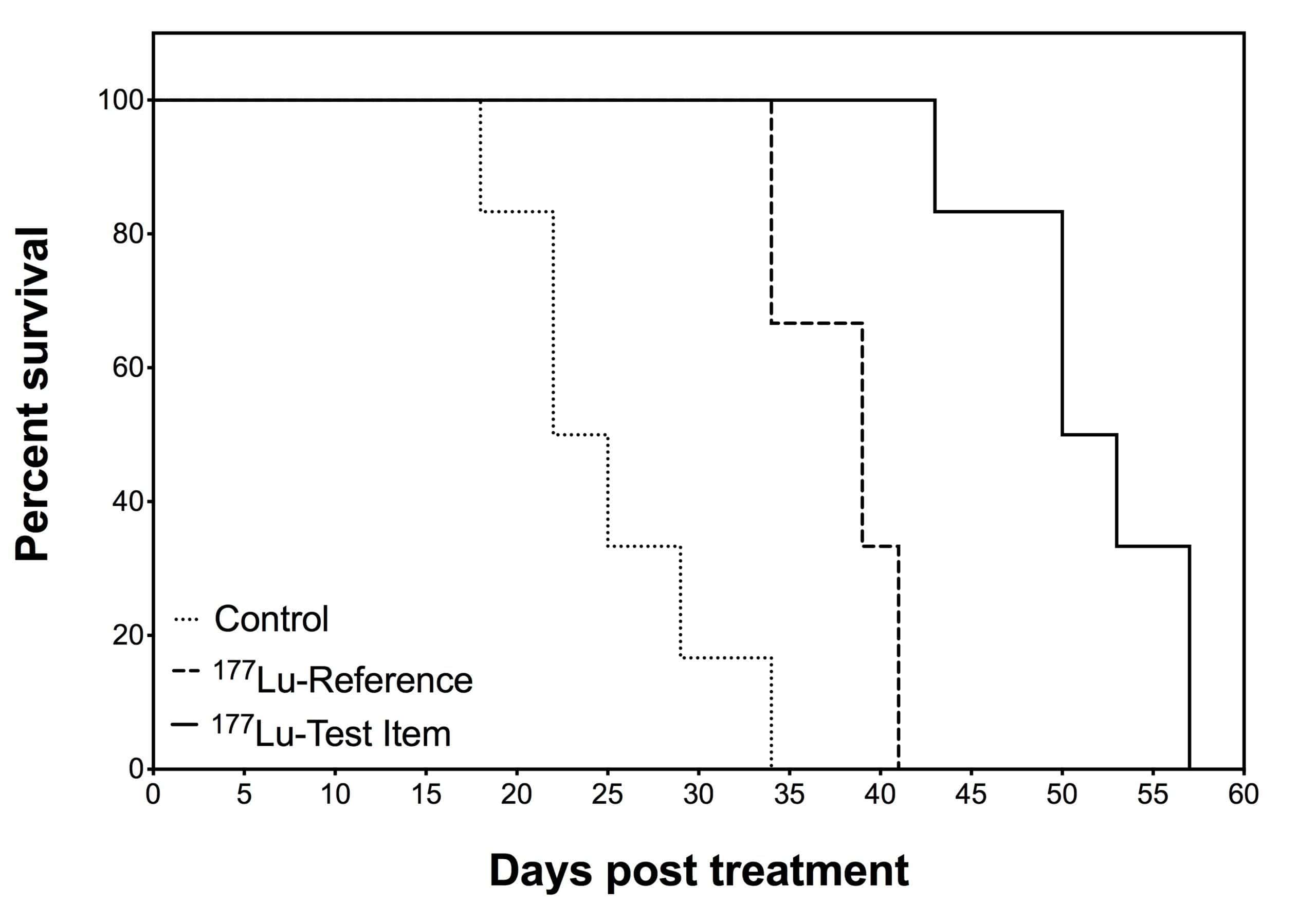
Typical study includes:
- A vehicle control arm
- Therapeutic radiopharmaceutical arms
- Up to 10 animals per arm
- Monitoring of animals twice a week over 45 days (or tumor size of 1500 mm3).
Any complementary arm including a reference test item can be added. Our efficacy studies can be tightly tailored and include various doses and injection routes. We can carry on up to 100 animals’ efficacy studies.
Dosimetry studies
Chelatec proposes dosimetry calculations based on pharmacokinetics and biodistribution data of your radiopharmaceutical. We follow the internal dosimetry schema of the Medical Internal Radiation Dose (MIRD) Committee.
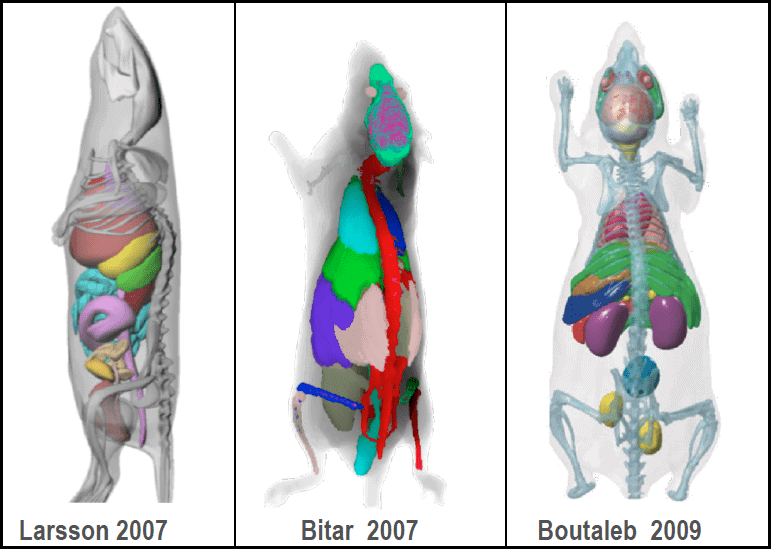
This approach is recognized as a method to perform absorbed dose calculation for internal emitters. In details, we use Monte Carlo based S-values calculated for a reference model to assess the delivered radiation dose to tissues/organs of the model.
We offer dosimetry calculations for Lutetium-177, Yttrium-90 as well as alpha emitters (Astatine-211, Actinium-225). Other radionuclides can be considered on special request.
Autoradiography of radiopharmaceuticals with tissue sections
For regulatory purposes, guidelines recommend that “any tests of toxicity, biological half-life, immunoreactivity and tissue cross-reactivity should be carried out on a form that is as close to the product to be administered to the patient as possible ”.

Chelatec proposes tissue sections autoradiography studies with your radiopharmaceutical (no “cold” non-radioactive labeled material required). Your radiopharmaceutical is directly incubated with tissue sections. Specific and non-specific binding will be evaluated with or without adding unlabeled conjugate.
Chelatec offers tissue sections autoradiography studies on:
- FDA / EMEA Panel of normal tissues
- Human cancer tissue sections
The development of radioimmunoconjugates for radioimmunotherapy is an innovative and attractive therapeutic field that requires multiple knowledge, skills and competencies. Mainly dedicated to oncology, these radiopharmaceuticals use either alpha, beta or gamma emitters.
With 20 years’ experience in the use of radioisotopes, our team will be glad to assist you in the design of the most appropriate study for the development of your radiopharmaceutical.